GLIA: Not Just Brain Glue!
Written by Sarah Kucenas, Ph.D., Associate Professor of Biology, Cell Biology and Neuroscience and Director of the Department of Biology Distinguished Majors Program, University of Virginia College and Graduate School of Arts & Sciences
The nervous system is the single most important organ system in the human body. It controls our ability to move, our breathing and heart rate, and is essential for survival and reproduction. It is the organ that makes us human, allowing us to interact with our environment and one another. However, this complex organ is susceptible to innumerable diseases and is incredibly fragile, making it easily damaged. Although intensely studied for hundreds of years, we still lack a complete picture of how the nervous system develops and what occurs during disease and injury. This is where my lab comes in!
Traditionally, the central nervous systems (CNS), which consists of the brain and spinal cord, and the peripheral nervous system (PNS), which consists of all of the motor and sensory nerves that innervate our skin, organs and muscles, have been thought of as two distinct halves of one organ system that are only connected by small bundles of motor and sensory axons. In each half of the nervous system reside specific subpopulations of neurons and glia. Although ignored for quite some time, glia, which is Greek for glue, are now known as essential players in neural development, synapse formation, plasticity, learning and memory, and disease. Recent work from my research group is redefining what we know about the separation of the two halves of the nervous system and we are forging a new field in neuroscience that investigates glial competition between cells in the CNS and PNS and the role these “battles” play in nervous system development and disease. Using Danio rerio (zebrafish) as a model system (Figure 1), my lab combines genetic and pharmacological perturbation, single cell manipulation, laser ablation/axotomy, small molecule screening and in vivo, time-lapse imaging to directly and continuously observe glial cell origins, behaviors and interactions in an intact, living system. By exploring the nature and role of glial competition during development and in injury/disease contexts, our work is not only laying the groundwork for a more fundamental understanding of the rules that form a functional nervous system, but is also shedding light on mechanisms that are perturbed in disease and could ultimately be harnessed therapeutically to treat neurodegenerative diseases.

Currently in the lab, we are pursuing a series of diverse projects aimed at characterizing the role of glia and glial-glial competition during nervous system development and disease. And it should be no surprise that we’re finding that glia are in fact, not just brain glue! Driven by motivated and determined scholars, my group consists of undergraduates, graduate students, post-doctoral fellows and research scientists tackling exciting and ground breaking studies. Some of them include:
1) The role of glial competition between cells in the CNS and PNS. Recently, we identified a novel class of glia that acts as a bouncer to keep distinct subpopulations of glia from mixing. We are currently characterizing these glial cells and the molecular mechanisms that mediate their interactions with neighboring glia. We are also investigating if we can manipulate the outcome of these glial battles in order to recruit specific populations of cells to regions of the nervous system that are affected by disease or injury in order to expedite repair.
2) Oligodendrocyte Competition. Oligodendrocytes myelinate CNS axons in order to facilitate rapid neurotransmission and provide protection from the surrounding environment. They are derived from oligodendrocyte progenitor cells (OPCs), which migrate throughout the developing CNS and become evenly distributed. In both developing and mature nervous systems, OPCs are highly motile cells with dynamic membrane processes that compete with neighboring OPCs for territory in the CNS (Figure 2). However, how competition between OPCs is molecularly controlled and how it influences the global distribution of OPCs within the CNS, are unknown. In these studies, we are uncovering the molecular mediators of OPC competition and investigating if we can manipulate them to enhance remyelination in diseases like Multiple Sclerosis.
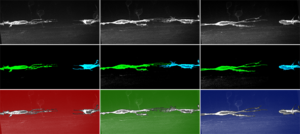
3) Nerve Regeneration. Previous studies from my lab discovered a novel, specialized class of glia known as perineurial glia that are essential mediators of spinal motor nerve regeneration. After nerve injury, perineurial glia not only aid in rapid clearance of nerve debris, they also provide an essential bridge across the injury gap which is required for axon regrowth. However, the cellular and molecular mechanisms that underlie how, when and why these glia bridge the injury, are poorly understood. We are currently identifying molecular mechanisms that mediate this essential perineurial glia migration following injury in order to enhance PNS regeneration after injury and disease. [See an example of this here: Movie 1.]
4) Is Duchenne Muscular Dystrophy (DMD) a disease of the nervous system? DMD has long been considered a disease of the muscle and nerve degeneration symptoms have been considered secondary to the muscle defect. However, nearly half of all DMD patients have cognitive defects. Recently, we created a zebrafish model of DMD to investigate the potential nervous system component of this disease. In our studies, we see severe nervous system defects that we hypothesize affect the severity and progression of this disease. The insight gained from these studies will not only lead to a more complete view of DMD, but will also provide the basis with which to propose novel diagnostic criteria and therapies for children with this debilitating disease.
5) Elucidating Glial Diversity: The PNS contains different types of glia with diverse functions including myelinating peripheral axons as well as protecting this organ from bacteria, viruses and injury. These glial types arise from different locations, have different developmental behaviors, acquire different morphologies and express unique combinations of genes. Although we are beginning to understand how peripheral glia develop and know a few of the genes important for their function, the full molecular programs that regulate their specification, migration, differentiation and function are unknown. Furthermore, there is increasing evidence that there is a far greater amount of glial diversity in the PNS than previously appreciated. In order to determine the level of glial heterogeneity in the PNS, we are interrogating the transcriptomes of several PNS glial subtypes and comparing them to CNS OPCs and oligodendrocytes. These studies will not only help us understand more about how peripheral glia develop, but will also help us investigate the distinct roles and molecular changes that occur in specific glial populations following nerve injury and demyelinating diseases.
For more information about the Kucenas Lab, our members and other projects, please visit: www.kucenaslab.com.
- Musings on National Violin Day
- Making the Promise Real: How a UN Tax Convention Can Fulfill the UNDHR’s Vision
- Having a Drink With Your Donkey: The Absurd in Antiquity
- UVA Club of Atlanta: Virtual Pilates Class
- UVA Club of Fairfield/Westchester: Cavs Care - Food Pantry Donation Drive
- UVA Club of Phoenix: Hoo-liday Party
