Tau Speed Bumps Protect Against Alzheimer’s Disease
Written by George S. Bloom, Ph.D., Professor of Biology, Cell Biology and Neuroscience and Director of the Undergraduate Program in Neuroscience, University of Virginia College and Graduate School of Arts and Sciences
Alzheimer’s disease (AD) attacks neurons (nerve cells) in the brain, and sometime in early 2013 it became the most expensive disease in the US. At a projected cost of nearly $260 billion for the current year, AD will cost American society more than cancer and heart disease in 2017. Unfortunately, unless new, disease-modifying drugs can be developed soon, the financial burden imposed by AD will continue to rise steadily and could increase as much as threefold by the middle of the century. There are many reasons that AD is still impossible to prevent or control clinically, and one of them is our primitive understanding of the underlying biology of the disease. With that knowledge gap in mind, my lab’s main focus for the past decade has been to define what happens at the very beginning to convert normal healthy neurons into AD neurons.
One of the most conspicuous features of AD is the aggregation [collection] of a neuronal protein called “tau”. When these aggregates are in the form of filaments, they constitute the well-known “neurofibrillary tangles” of AD brain, but less organized tau aggregates are also commonly found in neurons affected by AD. Although malfunctioning of tau is now widely recognized as a key step in the development of AD, remarkably little was known about how tau aggregates compromise the function of neurons. A recently published study1 from my lab is helping to solve that mystery.
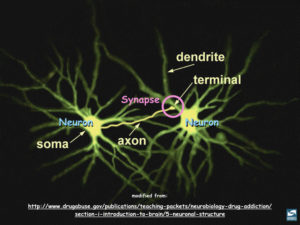
Building on work from other labs showing that extracellular tau filaments can cause the tau inside cells to aggregate, Dr. Eric Swanson, who completed his Ph.D. in my lab just a few months ago, found that extracellular tau “oligomers”, which are much smaller aggregates than tau filaments, cause the tau inside neurons not only to aggregate, but to spread from its normal location in axons into dendrites. Each neuron typically has one axon, a wire-like extension that can be as long as several centimeters in the human brain, and whose distant end sends signals to other neurons with which it makes contacts at “synapses”. Axons most commonly form synapses on dendrites, which are branched, tree-like parts of neurons that can extend up to several millimeters away from the neuronal cell nucleus. Networks of synapses are responsible for storing and processing information, and their demise accounts for the loss of memory and cognitive skills that afflicts AD patients What is important about Swanson’s observation is that invasion of tau into dendrites is known to cause synapses to fail and to kill neurons. Swanson’s work implies that extracellular oligomers, but not filaments made from tau, are what cause the tau inside neurons to invade dendrites, and thereby poison synapses and provoke neuron death. By extension, his work emphasizes that detection of tau oligomers might enable diagnosis of AD at a much earlier stage than is now possible, and that the oligomers represent potential drug targets for AD as well.
Swanson also made another important discovery as part of his study. The tau inside axons is normally stuck to microtubules, which are long intracellular fibers that serve as highways for transporting subcellular cargoes from place to place within the cell. Tau has been found by other labs to serve as speed bumps that regulate the pace at which cargoes can move along microtubules. Swanson discovered that when axonal tau aggregates after exposure of neurons to extracellular tau oligomers, axonal transport speeds up and cargoes move along microtubules for longer than normal distances. These results imply that aggregated axonal tau has lost its association with microtubules, causing the tau speed bumps to disappear. Since the health of the neuron depends on axonal transport occurring normally, Swanson’s results demonstrate that axonal transport, and overall neuron health by extension, can be poisoned by extracellular tau oligomers because they cause removal of tau speed bumps in axons. This conclusion further highlights the attractiveness of tau oligomers as therapeutic targets for AD.
1Swanson E, Breckenridge L, McMahon L, Som S, McConnell I and Bloom GS. 2017. Extracellular Tau Oligomers Induce Invasion of Endogenous Tau into the Somatodendritic Compartment and Axonal Transport Dysfunction. Journal of Alzheimer’s Disease 58(3): in press
- Stay on Track: Turning Resolutions into Results
- From “Jimmy Who?” to “What Would Jimmy Do?”
- Washington’s Bold Gamble: Christmas Day 1776
- Increasing Your Impact With Planned Giving
- UVA Club of Boston: UVA Men's Basketball Game Watches
- UVA Club of New Mexico: Cavs Care - Roadrunner Food Bank