Harnessing Immune Memory: mRNA Vaccines in COVID-19 and Beyond

 Jie Sun, PhD, Harrison Distinguished Teaching Professor of Diseases and International Health, University of Virginia School of Medicine
Jie Sun, PhD, Harrison Distinguished Teaching Professor of Diseases and International Health, University of Virginia School of Medicine
Samuel P. Young, Graduate Student, Microbiology, Immunology, and Cancer Biology, University of Virginia
I’ve spent much of my professional career in the field of viral immunology, studying the vastly complex responses elicited by the human immune system upon infection. My scientific focus has centered around how the immune system defends the body from viral infection, in order to ultimately improve patient treatment options. My journey has been significantly influenced by the University of Virginia, where I trained as a postdoc under the mentorship of the late Dr. Thomas Braciale. Coming full circle, Dr. Braciale persuaded me back to UVA in early 2022 as a faculty member.
As a viral immunologist, the COVID-19 pandemic has taught me a lot about the resiliency of humanity, especially concerning the unrelenting nature of scientific discovery. Given the dire need for rapid development of a COVID-19 vaccine, two decades’ worth of scientific discovery and ingenuity was expedited, culminating in the deployment of a novel ‘mRNA vaccine’ with world-saving efficacy.
Messenger RNA, or mRNA, serves as a blueprint for all cellular lifeforms to construct functional proteins. The idea behind an mRNA vaccine is that by providing our cells with a harmless blueprint derived from a pathogen like COVID-19, we can teach our immune system how to recognize and defend itself without causing disease. The foundational discoveries that enabled the rapid development of the COVID-19 mRNA vaccine originated at the University of Pennsylvania in the early 2000s, coincidentally during the time I was a graduate student there. It was there that Dr. Katalin Karikó and Dr. Drew Weissman discovered a method in which mRNA can be synthetically produced to promote long-lasting immune memory against potentially unseen pathogens, which led to their winning of the 2023 Nobel Prize in Medicine.
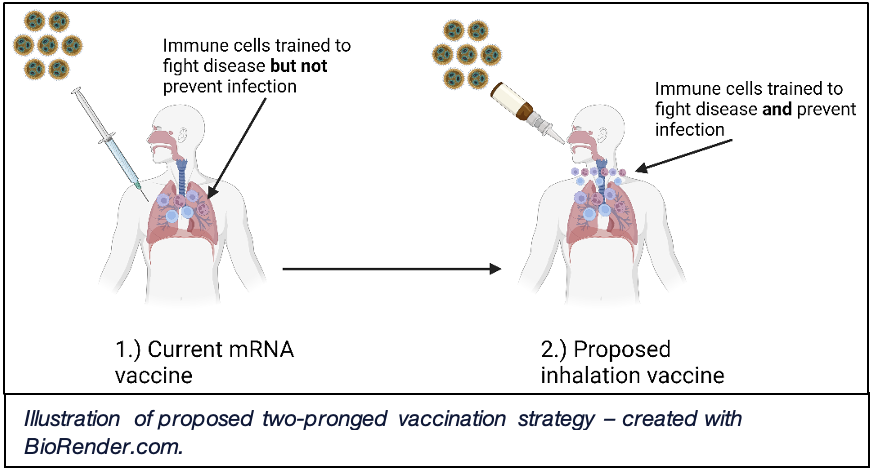
The key strength of the COVID-19 vaccine lies in its ability to reduce severe disease and, ultimately, death. However, a major focus of my research lab has centered around whether we can develop a vaccination strategy to prevent infection altogether. We like to compare this concept to a medieval castle, where the castle is the body and the soldiers are the cells of the immune system, valiantly defending their kingdom from invading pathogens. The current COVID-19 vaccine helps to train these cell-soldiers to fight against infection inside the castle walls but does not prevent breaching of the castle gate itself. Our plan is not only to train soldiers inside the gate to fight infection but also to deploy soldiers outside the gate to prevent infection altogether.
Using the cutting-edge mouse research facilities and technology available at UVA, we have harnessed a two-pronged vaccination strategy. An initial mRNA vaccine is first administered to lay the groundwork for a strong immune response, followed by an inhaled vaccination designed to mimic the normal COVID-19 viral route of entry through the airways. This is a vaccination strategy that has been used to great effect in India and China, and in our hands, we have found that the inhalation vaccine is able to enhance the immune system’s strength more specifically within the respiratory tract and lungs, leading to enhanced barrier protection (i.e. trained soldiers outside the castle gate).
These findings have led us to wonder whether other lung diseases, such as lung cancer, may benefit from a similar vaccination approach. Driving this project forward is my graduate student, Sam, so I thought he should be the one to explain it to you.
________________________________________________________________________
Adjacent to Jie’s central research focus, my interests lie in developing strategies to enhance the immune system’s ability to recognize and kill cancer cells. These interests arose during my undergraduate education, where I studied how repeated cellular mutations can drive cancer development. Speaking with Jie about my scientific interests, I realized the incredible potential his vaccination strategy could have in treating not just COVID-19, but other respiratory diseases like lung cancer.
 While vaccines are predominately used to treat infectious diseases, they can also be used to treat cancer. Cancer development is primarily driven through damage-induced DNA mutations, and subsequent mutant protein production. These mutant proteins can resemble normal proteins with often increased/decreased function or can be new proteins entirely. Therefore, we can develop vaccines that teach the immune system how to selectively kill cancer cells producing mutant proteins, while keeping healthy cells intact.
While vaccines are predominately used to treat infectious diseases, they can also be used to treat cancer. Cancer development is primarily driven through damage-induced DNA mutations, and subsequent mutant protein production. These mutant proteins can resemble normal proteins with often increased/decreased function or can be new proteins entirely. Therefore, we can develop vaccines that teach the immune system how to selectively kill cancer cells producing mutant proteins, while keeping healthy cells intact.
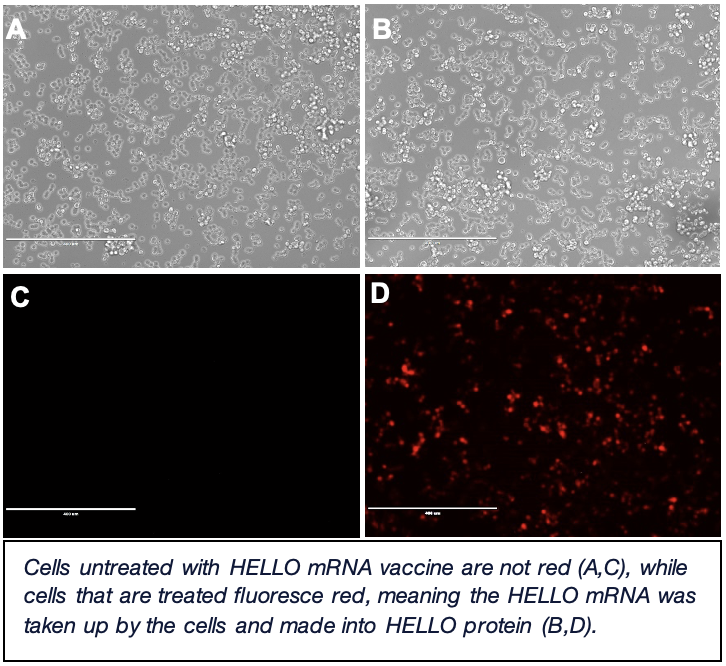
To apply our mRNA+inhalation vaccine strategy to cancer, we have a model system where cancer cells are administered into the lungs of mice. These cancer cells all produce the same mutated protein called “HELLO”. Currently, I’m developing an mRNA vaccine to specifically target the mutant HELLO protein. Part of HELLO is fluorescent red in color, allowing me to verify that my mRNA vaccine is correctly instructing cells (i.e. if I see red under a microscope, the cells are using the mRNA to make HELLO protein). Once developed, I will use this mRNA vaccine in combination with an inhalation vaccine to determine its therapeutic effect in cancer-burdened mice. Future work will entail figuring out how this vaccination strategy works at the molecular level to reduce cancer burden.
- A Revolution in the Air: The Wright Brothers Take to the Sky on December 17, 1903
- Musings on National Violin Day
- Making the Promise Real: How a UN Tax Convention Can Fulfill the UNDHR’s Vision
- UVA Club of Atlanta: Virtual Pilates Class
- UVA Club of Houston: Hoo-liday Party
- UVA Club of Fredericksburg: Hoo-liday Lights Tour